LYMPHIR is available for use.
Proven 36% ORR* with LYMPHIR in patients with relapsed or refractory CTCL1
Primary endpoint results1-3
Tumor response was evaluated by independent review committee assessment based on ISCL/EORTC GRS per Olsen et al, 2011.


Pivotal Study 302: This was an open-label, single-arm, phase III, multicenter trial of heavily pretreated adult patients with relapsed or refractory Stage I-III CTCL (N=69).1,2
ORR=CR+PR; 95% CI: 25%, 49%.2,3
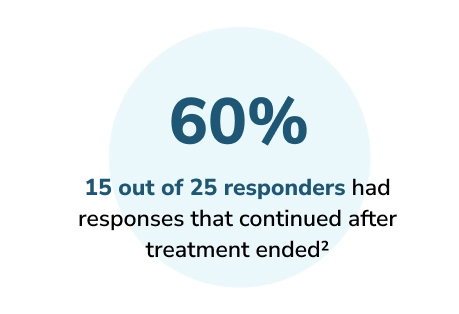
Secondary endpoint results1,2
Among responders (n=25):
mTTR: 1.4 months
(range: 0.7-5.6 months)
68% of patients saw responses in treatment cycles 1 and 2†
Serious adverse reactions occurred in 38% of patients who received LYMPHIR. Serious adverse reactions in >2% of patients included capillary leak syndrome (10%), infusion-related reaction (9%), sepsis (7%), skin infection (2.9%), pyrexia (2.9%), and rash (2.9%).
†Out of 69 patients, 64 patients who have baseline and at least 1 postbaseline mSWAT scores are included.2
Skin responses and pruritus improvement were both seen with LYMPHIR2
Secondary endpoint results
LYMPHIR demonstrated reduction in skin disease for 84% of patients
Waterfall plot for skin tumor burden (mSWAT score)†

Exploratory endpoint results2
LYMPHIR demonstrated clinically significant pruritus improvement
Pruritus improvement was defined as an absolute decrease of 20 mm from baseline maintained for ≥4 weeks (based on a 100-mm VAS). At baseline, 41 patients reported pruritus with a score of 20 or more (zero indicating no itch and 100 indicating worst possible itch).
Of all patients who experienced pruritus at baseline:
20 of 57 patients experienced ≥20% improvement
14 of 57 patients experienced ≥50% improvement


LYMPHIR was evaluated in CTCL patients who had tried multiple therapies to treat their disease1
Pivotal Study 302
This was an open-label, single-arm, phase III, multicenter trial of heavily pretreated adult patients with relapsed or refractory Stage I-III CTCL (N=69). LYMPHIR 9 mcg/kg was given as an IV infusion daily from Day 1 through Day 5 of each 21-day cycle for up to 8 cycles. Patients who showed clinical benefit could continue LYMPHIR treatment beyond 8 cycles at the discretion of the treating physician. The median number of LYMPHIR cycles was 6 (range: 1-42). Patients were excluded if they had significant cardiac disease or uncontrolled infections.1,2
Baseline patient characteristics1
| Age (median) | 64 years (range: 28-87 years) |
|---|---|
| Racial demographics | 73% White 19% Black or African American 14% Hispanic or Latino 1% Asian |
| Sex | 65% Male 35% Female |
Patients in the study had a median of 4 prior therapies (range: 1-18) including both skin-directed and systemic therapies1
Photodynamic therapy (56%)
Total skin electron beam therapy (42%)
Systemic retinoids (49%)
Methotrexate/pralatrexate (49%)
Histone deacetylase inhibitor (35%)
Brentuximab vedotin (26%)
Mogamulizumab (12%)
CTCL disease stage1


Premedication was required in Cycles 1-3 and optional thereafter. These premedications included but were not limited to2:
Acetaminophen 650 mg by mouth to prevent fever and chills
Diphenhydramine 25 mg IV push to prevent hives, itching, rash, shortness of breath, and chills
Antiemetic agents
Hydration with 250 mL to 500 mL normal saline IV before and after each LYMPHIR infusion
NCCN category 2A recommendation4§
Denileukin diftitox-cxdl (LYMPHIR) is recommended as an NCCN Category 2A§ treatment option for Stage IB-III mycosis fungoides by the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®).4
See the NCCN Guidelines for detailed recommendations, including other treatment options. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
‡Not estimable by Kaplan-Meier method.1
§Category 2A is based upon lower-level evidence. There is uniform NCCN consensus (≥85% of the Panel) that the intervention is appropriate.4
EORTC=European Organization of Research and Treatment of Cancer; GRS=Global Response Score; ISCL=International Society for Cutaneous Lymphomas; mDOR=median duration of response; mSWAT=modified Severity Weighted Assessment Tool; mTTR=median time to response; VAS=visual analog scale.
References: 1. LYMPHIR. Prescribing information. Citius Oncology, Inc.; 2024. 2. Data on file. Citius Oncology, Inc. 3. Olsen EA, Whittaker S, Kim YH, et al. Clinical endpoints and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society of Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598-2607. doi:10.1200/JCO.2010.32.0630 4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Primary Cutaneous Lymphomas V.3.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed November 13, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
