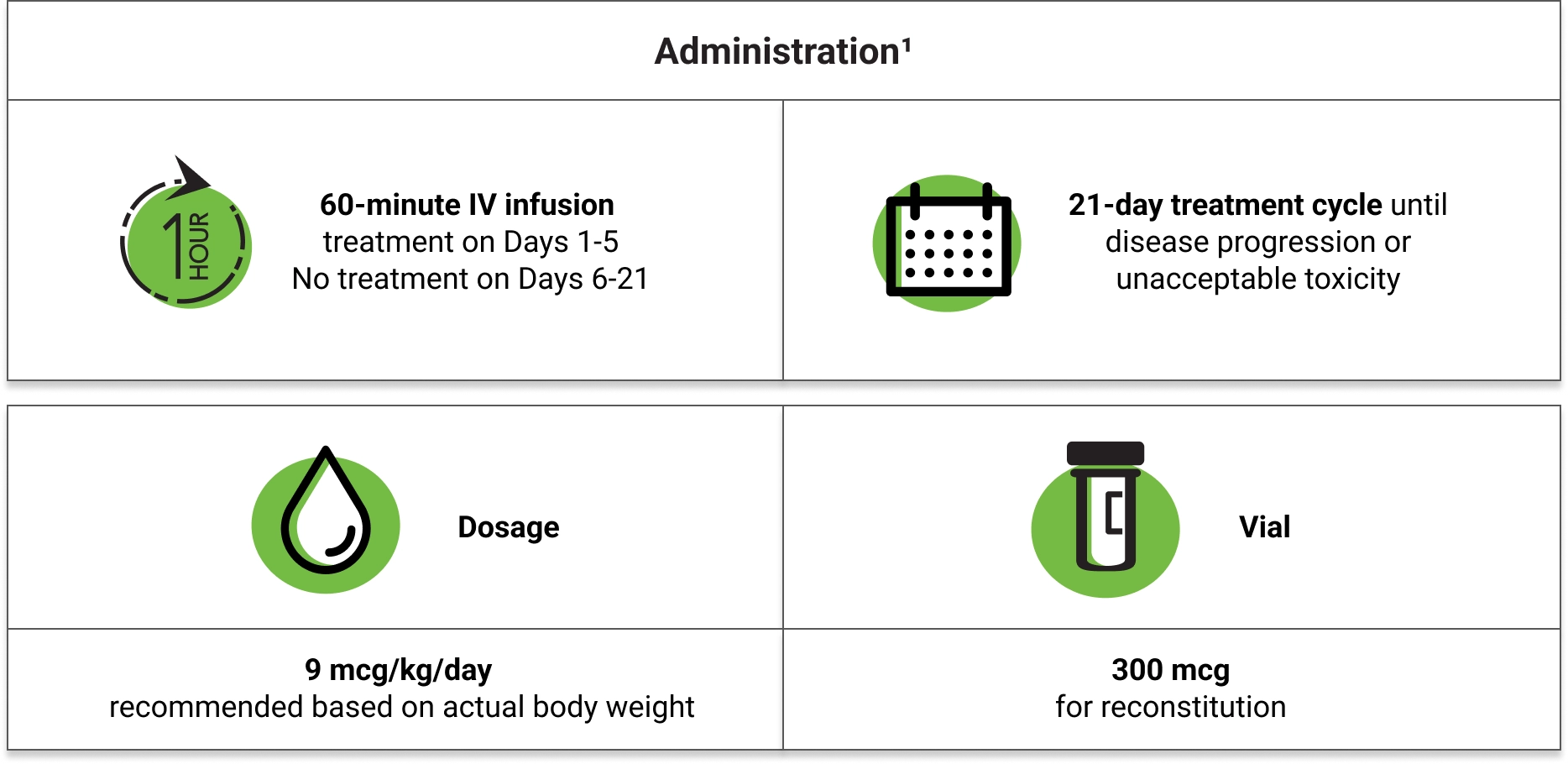
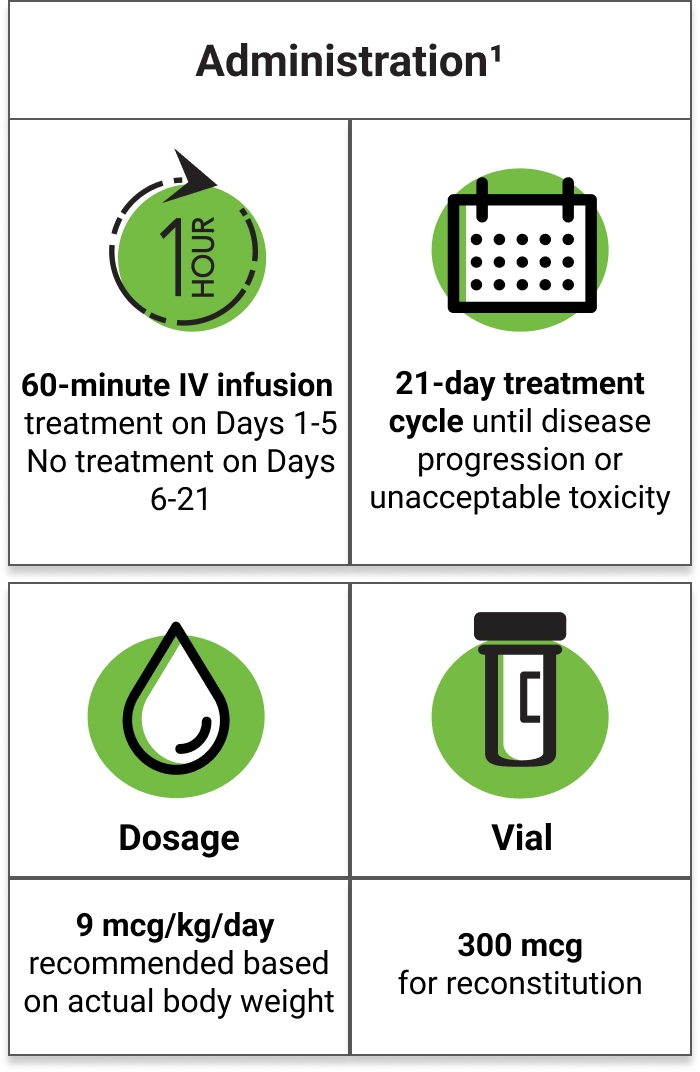
Capillary Leak Syndrome
LYMPHIR can cause capillary leak syndrome (CLS), including life-threatening or fatal reactions. CLS was defined in the clinical trials as the occurrence of at least 2 of the following symptoms at any time during LYMPHIR therapy: hypotension, edema, and serum albumin <3 g/dL. These symptoms were not required to occur simultaneously to be characterized as CLS.
As defined, CLS occurred in 27% of patients in the pooled population across 3 clinical trials, including 8% with Grade 3. There was one (0.8%) fatal occurrence of CLS. Of the patients with CLS, 22% had recurrence. The majority of CLS events (81%) occurred within the first 2 cycles of treatment. The median time to onset from Cycle 1, Day 1 was 6.5 days (range: 1 to 77), the median duration of CLS was 14 days (range: 2 to 40), and 75% of patients had resolution.
Regularly assess patients for weight gain, new onset or worsening of edema, dyspnea, and hypotension (including orthostatic changes). Monitor serum albumin levels prior to the initiation of each cycle of therapy and more often as clinically indicated.
Withhold, reduce dose, or permanently discontinue based on severity. If LYMPHIR is withheld, resume LYMPHIR following resolution of CLS and when serum albumin is ≥3 g/dL.
Visual Impairment
LYMPHIR can cause serious visual impairment, including changes in visual acuity and color vision. In the pooled population across 3 clinical trials, visual impairment occurred in 9%, with Grade 1 in 8% and Grade 2 in 1%. The most commonly reported symptom was blurred vision. Of the patients with visual impairment, 67% had resolution of their visual impairment.
Perform baseline ophthalmic examination and monitor as clinically indicated. If patients experience symptoms of visual impairment, such as changes in visual acuity, changes in color vision, or blurred vision, refer for ophthalmologic evaluation. Withhold LYMPHIR until visual impairment resolves or permanently discontinue based on severity.
Infusion-Related Reactions
LYMPHIR can cause serious infusion-related reactions. Infusion-related reactions were reported in 69% of patients in the pooled population across 3 clinical trials of patients who received LYMPHIR, with Grade 3 infusion-related reactions in 3.4%. Eighty-three percent of infusion-related reactions occurred in Cycles 1 and 2. The most common symptoms included nausea, fatigue, chills, musculoskeletal pain, vomiting, fever, and arthralgia.
Premedicate patients for the first 3 cycles prior to starting a LYMPHIR infusion. Monitor patients frequently during infusion. For Grade 2 or higher infusion reactions, premedicate at least 30 minutes prior to each subsequent infusion with a systemic steroid for at least 3 cycles. Interrupt or discontinue LYMPHIR based on severity. Institute appropriate medical management.
Hepatotoxicity
LYMPHIR can cause hepatotoxicity. In the pooled safety population, elevated ALT occurred in 70% of patients, with Grade 3 ALT occurring in 22%; elevated AST occurred in 64% of patients, with Grade 3 AST elevation occurring in 9%. For Grade 3 events, median time to onset was 8 days (range: 1 to 15 days); median time to resolution was 15 days (range: 7 to 50 days); all cases of Grade 3 ALT or AST elevations resolved. Elevated total bilirubin occurred in 5% of patients, with Grade 3 occurring in 0.9%.
Monitor liver enzymes and bilirubin at baseline and during treatment as clinically indicated. Withhold, reduce dose, or permanently discontinue LYMPHIR based on severity.
Embryo-Fetal Toxicity
Based on its mechanism of action, LYMPHIR can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to the initiation of LYMPHIR. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment and for 7 days following the last dose of LYMPHIR.
Adverse Reactions
In the pooled safety population, the most common (≥20%) adverse reactions, including laboratory abnormalities, were increased transaminases (70%), albumin decreased (53%), nausea (40%), edema (35%), hemoglobin decreased (34%), fatigue (30%), musculoskeletal pain (26%), rash (23%), chills (22%), constipation (22%), pyrexia (21%), and CLS (20%).
Use in Specific Populations
Lactation: Advise women not to breastfeed during treatment with LYMPHIR and for 7 days after the last dose.
You may report side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. You may also report side effects to Citius Oncology at 1-844-459-6744.
INDICATIONS AND USAGE
LYMPHIR (denileukin diftitox-cxdl) is indicated for the treatment of adult patients with relapsed or refractory Stage I-III cutaneous T-cell lymphoma (CTCL) after at least one prior systemic therapy.
Please see full Prescribing Information for additional Important Safety Information, including Boxed Warning.